RETRO-C Cancer Biomarker Assays
Rapid Single-Plex Detection
Oncology Screening and Monitoring
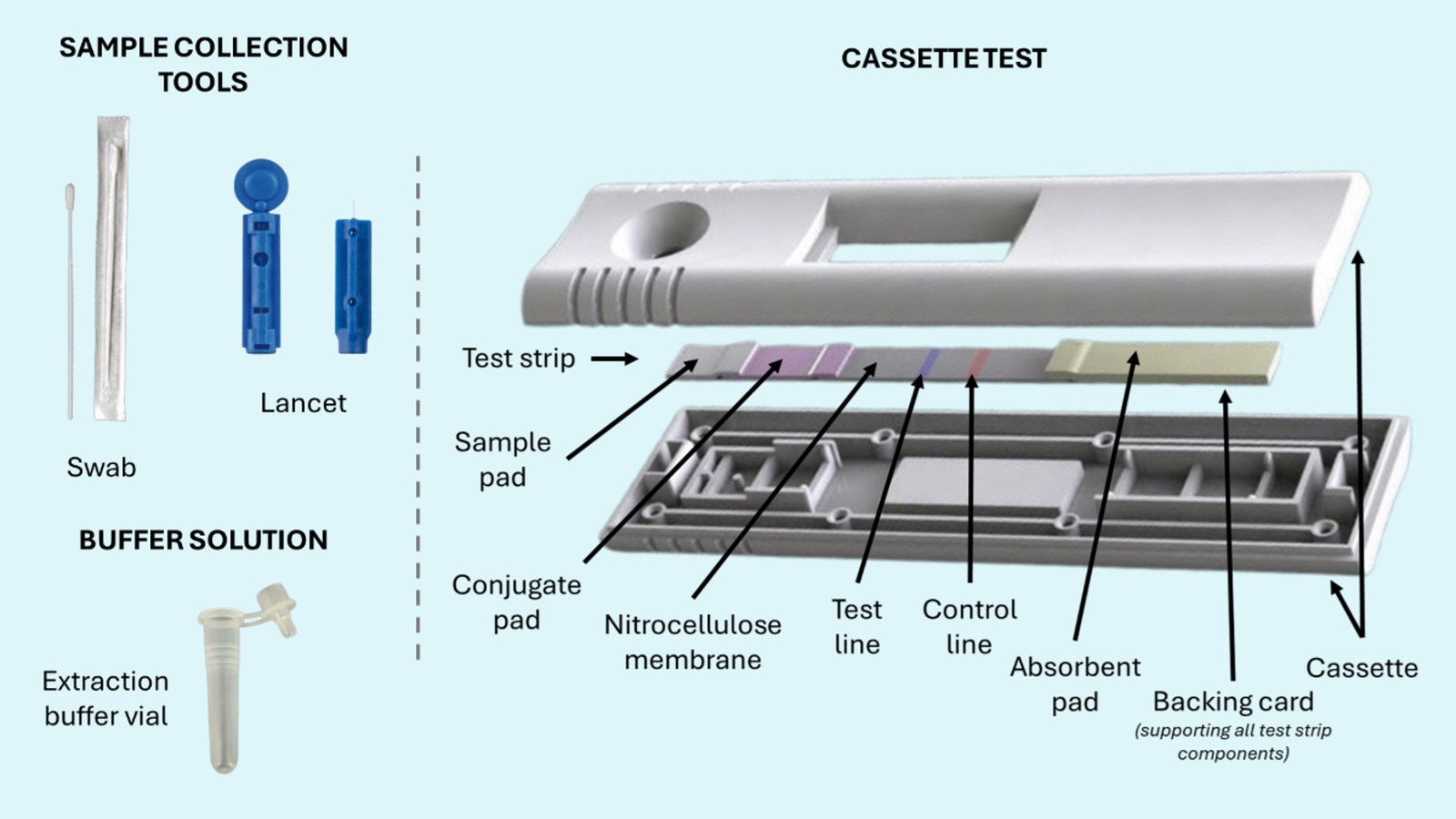
Each RETRO-C assay is designed as a standalone test, allowing healthcare providers to select biomarkers most relevant to individual patient needs and clinical contexts. The single-plex format ensures maximum sensitivity and specificity for each analyte, avoiding the compromises sometimes necessary in multiplex panels. Sample types supported include whole blood, serum, and plasma, accommodating standard clinical specimen collection protocols.
Available Biomarkers:
Ideal For
Oncology clinics, primary care screening programs, rural healthcare facilities, mobile health units, patient monitoring during chemotherapy, and early detection initiatives in resource-limited settings. The assays support screening programs targeting high-risk populations and provide accessible options for longitudinal monitoring.
