About Us
Who We Are
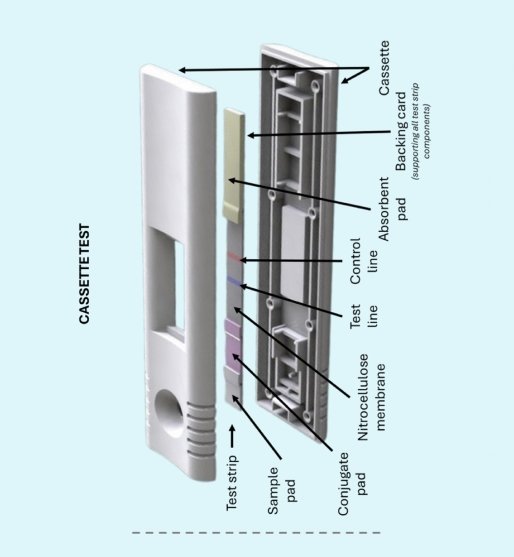
Pioneering Accessible Diagnostic Excellence
Our journey began with a deep understanding of the critical need for reliable, rapid diagnostic solutions in resource-varied settings. We recognized that while molecular diagnostics offer exceptional accuracy, they often require infrastructure and timelines incompatible with point-of-care decision-making. Conversely, traditional rapid tests provide speed but lack quantitative precision. Retroindica set out to solve this challenge.
Today, we operate at the intersection of immunoassay science, nanotechnology, microfluidics, and artificial intelligence. Our flagship platforms—RETRO-RV for multiplex respiratory virus detection and RETRO-C for single-plex cancer biomarker assays—represent years of research, development, and clinical validation. These products embody our commitment to scientific rigor, manufacturing excellence, and practical healthcare solutions.