About Us
Translational Diagnostics—From Bench to Bedside
OUR SERVICES
Explore Our Services
Simultaneous detection of SARS-CoV-2, Influenza A/B, and RSV on a single test strip for rapid differential diagnosis at point-of-care.
Rapid detection of cancer biomarkers including CEA, AFP, PSA, CA-125, CA-15-3, CA-19-9, and HE-4 for screening and monitoring.
Transform visual test results into precise semi-quantitative data using AI-powered camera-based readers and secure cloud analytics.
Comprehensive validation services including analytical and clinical studies to accelerate your diagnostic product to market.
End-to-end CDMO services from feasibility studies through scale-up manufacturing and regulatory documentation support.
Comprehensive training programs and responsive technical support to ensure optimal performance of our diagnostic solutions.
OUR BLOG
Latest Insights & Updates

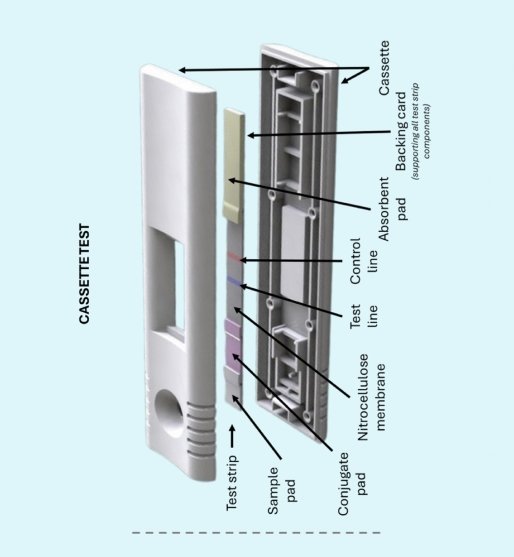
Lateral flow assays have quietly revolutionized point-of-care diagnostics over the past four decades. What began as simple home pregnancy tests has evolved into sophisticated diagnostic platforms.

Cancer biomarkers have transformed oncology practice over the past three decades. Understanding when, how, and why to use rapid biomarker tests can make a significant difference in patient outcomes.

The integration of cloud-based analytics with lateral flow diagnostics represents a paradigm shift in how point-of-care test results are interpreted, stored, and utilized for clinical decision-making.